Project Details
Background
According to the World Health Organisation 260,000,000 people of all ages worldwide suffer from depression. Depression is the leading global cause of disability, lost days at work, and missed opportunities in education. Furthermore, the WHO estimates that more than three quarters of sufferers from depression receive no treatment. In many cases this is due to public policies that favour drugs instead of treatment when both have important roles to play. In the developing world it is the result of a lack of clinical resources. More patient involvement in strategies to manage the condition is essential to achieving better outcomes for them. One area of innovation is the development of technologies that allow patients to record their state of mind regularly and to share this with clinicians. When treated as data such information can advance clinical knowledge and provide opportunities for research into treatments.
Challenge
Blueridge Technologies asked Greenfinch to develop integrated smartphone and web applications to enable patients diagnosed with depression or bipolar disorders to record and monitor their daily mood.
Outcome
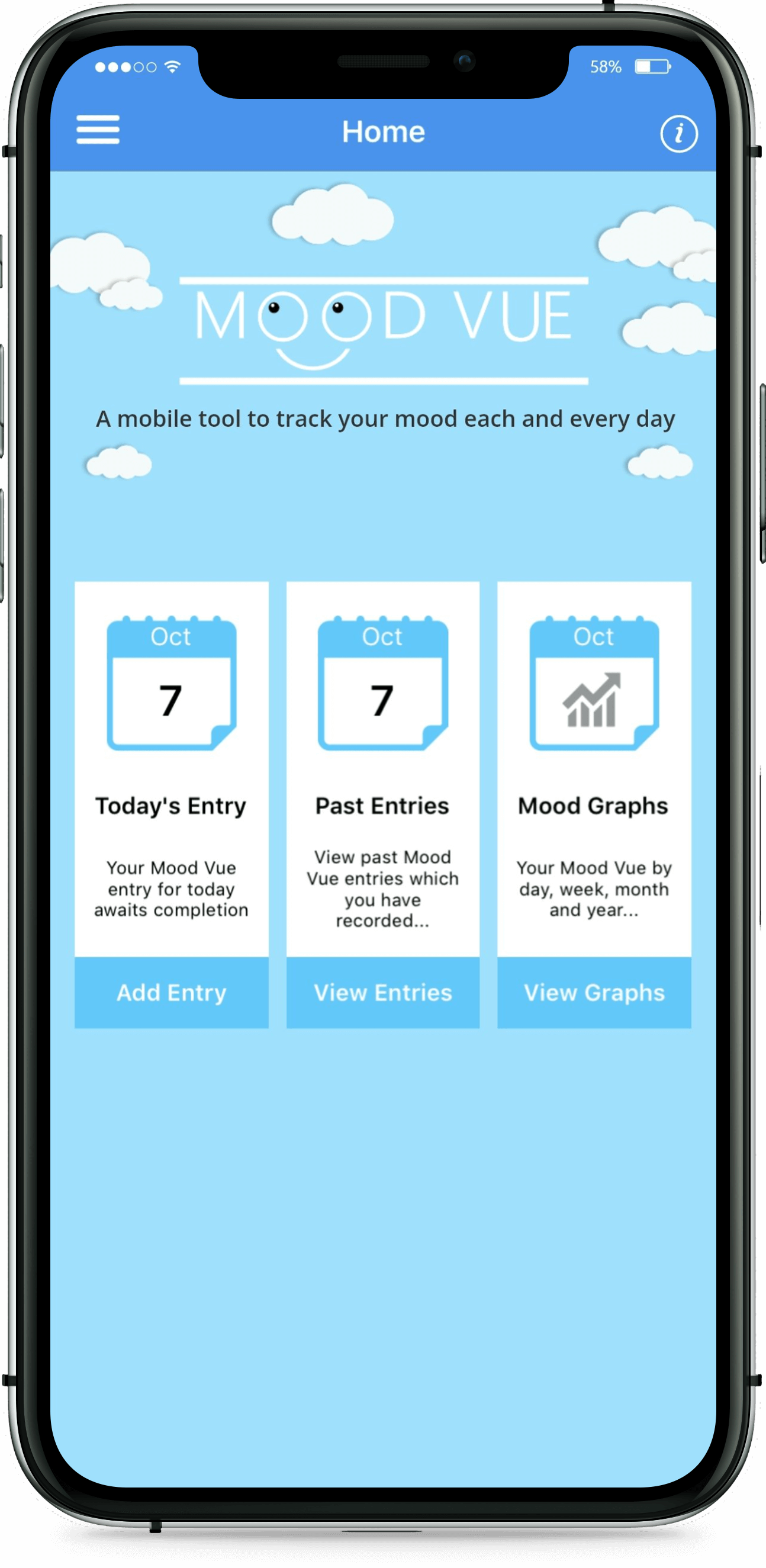
Greenfinch developed the Daily Mood Diary app, together with a web interface that allows clinicians to transfer questionnaires to it. The app is in both iOS and Android. Users can opt to answer either a short or more extended questionnaire. They can also set reminders to complete the questionnaire at particular times. Clinicians are able to send multiple choice questions to the app. The patient’s mood is measurable based on replies which record thoughts, feelings, sleep, energy, level of activity, physical pain, and mood variation. Previous results and review statistics are available to the user via the app, and there is an option to view previous results graphically. Users can then share this data with their clinician.
BlueBridge Technologies created the relevant Design and Development Documentation for the DMD software under its ISO 13485 and IEC 62304 accredited Quality Management System and provided it to the client for use in their technical file.
Technology Used
Xamarin Mobile

Microsoft Azure

C Sharp

Microsoft Server

Microsoft Framework

Jenkins Deployment